By Converting Fat Cells into Stem Cells That Can Help That Vital Organ Repair Itself, Says One UH Researcher

Professor Robert Schwartz: ‘We’ll do any technology that allows us to find answers.’There has been little time to savor the successes of the moment as Robert Schwartz
and members of his lab push relentlessly toward their goal: clinical trials he expects
will show that common fat cells, treated with two proteins, can convert quickly into
stem cells capable of helping damaged heart muscle repair itself.
That stunning development could come within a year, a huge step forward in the treatment of heart disease, while also offering promise in a range of muscular, neurological and other disorders.
But Schwartz’s impact reaches beyond his own work. He has been involved in one of the most important trends in research during the past decade, as scientists move out of their individual laboratories to collaborate across disciplines and across institutions, doing whatever it takes to solve complicated problems.
“Contemporary research is team research. It’s interdisciplinary,” he says. “Research
goes faster when you can interact with terrific scientists. You can ask basic questions
that lead to clinical applications.”
Schwartz, Cullen Distinguished Professor of biology and biochemistry, arrived at the
University of Houston in late 2009, recruited from Texas A&M Health Science Center
to help build the University’s biomedical sciences research initiative. He is also
director of the Stem Cell Engineering Laboratory at the Texas Heart Institute, a role
that helps in building a strong network of researchers from across the Texas Medical
Center.
His lab, the Center for Molecular Medicine and Experimental Therapeutics, is a sweeping
space on the fourth floor of the Science and Engineering Research Center (SERC), where
more than two dozen researchers and graduate students also work on drug discovery,
including new generation of rho-kinase inhibitors, noncoding RNAs that alter cell
lineages and the origin of cancer stem cells.
“We’re not just a single-focused group,” he explains. “We’ll do any technology that
allows us to find answers. We’re totally unafraid.”
Schwartz is recognized for launching the field of myogenesis, or muscle development, in 1981 by defining the regulatory paradigm in which nonmuscle contractile proteins are switched off during muscle differentiation and replaced by muscle-specific contractile protein isoforms.
In the ensuing 33 years, he has continued to make significant discoveries, many of them focused on developmental and genetic aspects of congenital heart disease.
Creating Cardiac Stem Cells
His current major project involves converting somatic cells into cardiac stem cells. The work, now in small animal testing, involves reprogramming fat stem cells to function as one of three types of cells – cardiac cells, smooth muscle cells or vascular cells, depending on where they are implanted.
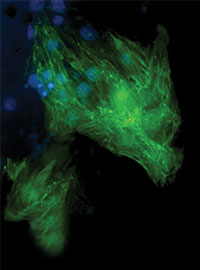
Cardiac progenitor cells are transformed into cardiac muscle cells, potentially allowing
a damaged heart to heal itself.The concept, once it moves to patients, would be to remove the fat cells via liposuction
and centrifuging away the fat cells from their stem cells. Next the fat stem cells
are incubated with factors that convert them to cardiac progenitor cells and then
inserted into an area of the heart muscle that has been damaged by a heart attack.
This allows the stem cells to begin replacing dead heart tissue with healthy muscle
and cardiovascular tissue.
Working in collaboration with scientists from the Texas Heart Institute, Schwartz’s team reported in 2012 that they had successfully reprogrammed human skin cells to function as heart muscle cells. They used two proteins, ETS2 and MESP1, to see if the proteins would work together to transform skin cells into heart cells. They were pleased to find the treated skin cells began to convert into immature heart muscle cells within two days.
The team now is working with fat cells, rather than the skin cells from its original research, but using the same transformational proteins, Schwartz says. Fat cells don’t convert more quickly – both skin cells and fat cells take about two days to convert to stem cells – but using fat cells as a base produces more stem cells.
“Simply put, it’s a more robust system,” he says.
Although Schwartz and his team are not the only researchers to convert adult cells into stem cells, he says they were the first to demonstrate the ability to convert human skin cells into muscle cells with the fewest factors. The team, along with the University of Houston, the Texas Heart Institute and Texas A&M University, was recently granted a U.S. patent covering the proteins, technology and viruses used to convert any cell to cardiac progenitor cells.
Advantage Over Embryonic Stem Cells
Both skin and fat cells have another advantage compared with embryonic stem cells, once considered the most promising field but long mired in controversy, making it more difficult for scientists to pursue the potential rewards.
Using skin or fat cells that can be converted to stem cells avoids moral and ethical issues inherent in the use of embryonic stem cells, Schwartz says, as well as concerns that embryonic stem cells may stimulate cancer growth. And, he notes, they are also more readily available.
But for all the focus on the promising use of these fat cells to repair heart damage after a heart attack, that’s only part of what is going on at the Center for Molecular Medicine and Experimental Therapeutics.
MESP, one of the factors used to convert the fat cells to stem cells, can also drive blood and muscle development. Recently, Li Chen, one of five faculty members working at the center, has focused on MESP’s role in embryonic pancreas formation, perhaps leading one day to repairing damaged pancreas tissue. Other faculty members working there include Yu Liu, Steven Bark, David Stewart and Peggy Zhang.
Important Collaborations
There also are other researchers, both inside UH – including biomedical engineer Ravi Birla and James Briggs, associate professor of biology and biochemistry – and outside, including developmental biologist Dr. James Martin from Baylor College of Medicine and Dr. James Willerson, president of the Texas Heart Institute, whom Schwartz cites as being important to collaborations that push the work forward.
The fat cells-to-cardiac stem cells work is an example of what researchers call the “lab bench to bedside” phenomenon, and Schwartz is delighted to see that it is really happening.
“This is a very important year for us,” he says, “one in which we can actually apply our research to clinical studies. Developing a practical and effective method to generate new heart muscle cells for cardiac repair is one of the truly great needs facing us today. And the broader implications of our research are ever more promising. We believe we’re on a successful path to achieve this – and what could be more exciting than that?”
See PDF from Research & Innovation Magazine
- Jeannie Kever, UH’s Research and Innovation Magazine