Faculty Profile
 Ognjen Miljanic
Ognjen Miljanic
Moores Professor
Department of Chemistry
Office: SERC, 5028
Contact: miljanic@uh.edu - 832-842-8827
Education: Ph.D., University of California, Berkeley, 2005; Diploma, University of Belgrade, Serbia, 2000
Postdoc University of California, Los Angeles, 2005-2008
Self-Sorting of Dynamic Combinatorial Libraries
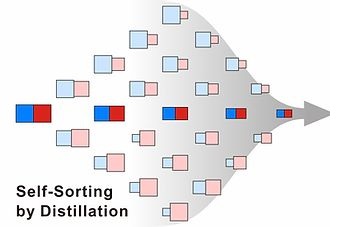 Nature is a master of chemoselectivity: in a typical biological cell, hundreds of
reactions occur simultaneously, but without any interference, in a complex "soup"
of biological precursors. We are seeking to replicate this behavior in complex libraries
of synthetic compounds known as dynamic combinatorial libraries (DCLs). We have shown
that libraries of as many as 100 members can be reduced in complexity to just a handful
of discrete compounds, produced in high purities.
Nature is a master of chemoselectivity: in a typical biological cell, hundreds of
reactions occur simultaneously, but without any interference, in a complex "soup"
of biological precursors. We are seeking to replicate this behavior in complex libraries
of synthetic compounds known as dynamic combinatorial libraries (DCLs). We have shown
that libraries of as many as 100 members can be reduced in complexity to just a handful
of discrete compounds, produced in high purities.
Reviewed in Chem 2017, 2, 502–524
Porous Materials and Porous Molecular Crystals
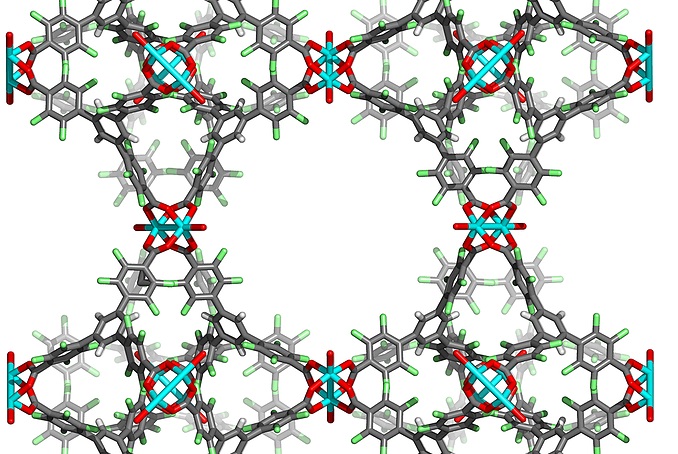 Porous materials have a plethora of application in industry, mostly related to energy
technologies. We are particularly interested in the synthesis of metal-organic and
fully organic porous frameworks through the use of sophisticated organic precursors
that assemble into porous structures either on their own, or upon coordination to
metals. These frameworks have found applications in binding of fluorocarbons, Freons
(CFCs) and fluorinated anesthetics.
Porous materials have a plethora of application in industry, mostly related to energy
technologies. We are particularly interested in the synthesis of metal-organic and
fully organic porous frameworks through the use of sophisticated organic precursors
that assemble into porous structures either on their own, or upon coordination to
metals. These frameworks have found applications in binding of fluorocarbons, Freons
(CFCs) and fluorinated anesthetics.
Reviewed in Org. Mater. 2019, 1, 19–29
Cyclobenzoins
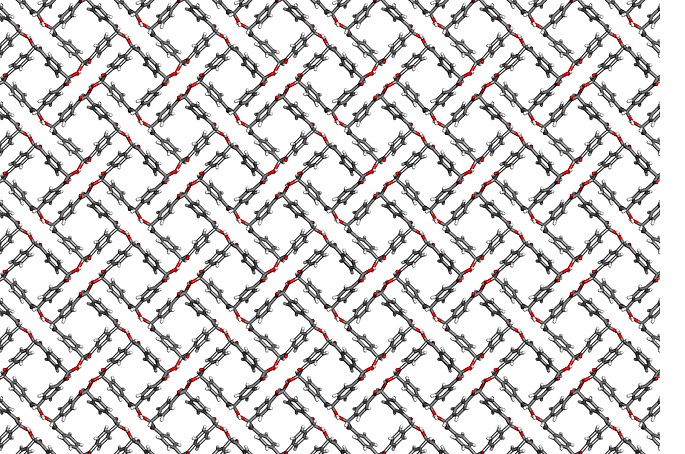 In 2015, we have discovered that benzoin condensation of small aromatic dialdehydes
leads to the generation of cyclic trimeric and tetrameric adducts, which we dubbed
cyclobenzoins. These new macrocycles are characterized by exceedingly simply synthesis,
rigid cavities, and rich chemistry that harks back to the early 19th century days
of benzoin condensation.
In 2015, we have discovered that benzoin condensation of small aromatic dialdehydes
leads to the generation of cyclic trimeric and tetrameric adducts, which we dubbed
cyclobenzoins. These new macrocycles are characterized by exceedingly simply synthesis,
rigid cavities, and rich chemistry that harks back to the early 19th century days
of benzoin condensation.
Reviewed in Chem. Commun. 2018, 54, 11989–11997
Rigid Aromatic Fluorophores
 We have developed cross-conjugated X-shaped fluorophores based on benzobisoxazole
and benzimidazole nuclei. In these molecules, appropriate substitution of the x- and
y-axes results in the spatial isolation of FMOs, leading to predictable and useful
sensing response. These systems have been utilized as sensors for carboxylic and boronic
acids, phenols, amines, and various anions. More recently, our interest have expandedto
the aggregation-induced emission in precursors to porous molecular crystals.
We have developed cross-conjugated X-shaped fluorophores based on benzobisoxazole
and benzimidazole nuclei. In these molecules, appropriate substitution of the x- and
y-axes results in the spatial isolation of FMOs, leading to predictable and useful
sensing response. These systems have been utilized as sensors for carboxylic and boronic
acids, phenols, amines, and various anions. More recently, our interest have expandedto
the aggregation-induced emission in precursors to porous molecular crystals.
Reviewed in Acc. Chem. Res. 2014, 47, 2074–2083
The Miljanić lab relies on supramolecular and synthetic chemistry as its key tools. Synthesis is used in the broad sense of the word, encompassing organic, coordinative inorganic, and organometallic preparations. Spectroscopy, crystallography, and materials characterization are the most relevant analytical tools. Students and postdoctoral researchers in the Miljanić group receive detailed guidance in scientific writing and visualization. This diversified training is intended to prepare them for challenges in both academic and a variety of industrial work environments.
- 2018 - Max Kade Foundation Fellowship, Ruprecht-Karls-Universität Heidelberg
- 2016 - Honorary Membership, Israel Chemical Society
- 2014 - UH John C. Butler Teaching Excellence Award
- 2014 - UH Award for Excellence in Research and Scholarship
- 2013 - Cottrell Scholar Award
- 2013 - Early Excellence Profile—Journal of Physical Organic Chemistry
- 2012 - UH Teaching Excellence Award
- 2012 - NSF CAREER Award
- 2011 - Thieme Chemistry Journal Award
- 2010 - ACS Greater Houston Section Younger Chemist of the Year Award
- 2008 - Postdoctoral Research Excellence Award—UCLA
- 2000 - Student of the Generation Award—University of Belgrade
- 1999 - Serbian Academy of Arts and Sciences Fellow