Faculty Profile
 Allan Jacobson
Allan Jacobson
Robert A. Welch Chair of Science
Department of Chemistry
Office: SR1, 14
Contact: ajjacob@uh.edu - 713-743-2785
Education: Ph.D., New College, Oxford, 1969; M.A., New College, Oxford, 1969; B.A., St. Catherine's College, Oxford, 1966
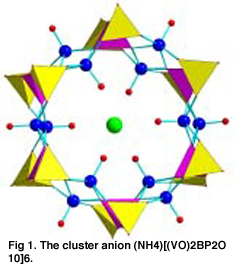 The synthesis and properties of transition metal oxide systems with layered or framework
structures are one focus of my research. We study the synthesis of new compounds with
asymmetric layer structures, open framework structures that can absorb molecules,
and new synthetic techniques including hydrothermal electro-crystallization and reactions
in ionic liquids. Asymmetric layer structures that we have investigated include the
compounds A2(MO3)3XO3 where A = alkali metal, M = Mo, W and X = Se or CH3P-. All are inherently non-centrosymmetric and show nonlinear optical properties.
We also use hydrothermal synthesis and coordination chemistry to construct new open
framework structures. Recent examples include assembly of cluster anions such as [V2P2BO10]6 18- into framework structures, the synthesis of Ni(CN)4(Ph3Sn)2.xS, a coordination system with a very low-density framework, and single crystal growth
and structure refinement of the titano-silicate ETS-10.
The synthesis and properties of transition metal oxide systems with layered or framework
structures are one focus of my research. We study the synthesis of new compounds with
asymmetric layer structures, open framework structures that can absorb molecules,
and new synthetic techniques including hydrothermal electro-crystallization and reactions
in ionic liquids. Asymmetric layer structures that we have investigated include the
compounds A2(MO3)3XO3 where A = alkali metal, M = Mo, W and X = Se or CH3P-. All are inherently non-centrosymmetric and show nonlinear optical properties.
We also use hydrothermal synthesis and coordination chemistry to construct new open
framework structures. Recent examples include assembly of cluster anions such as [V2P2BO10]6 18- into framework structures, the synthesis of Ni(CN)4(Ph3Sn)2.xS, a coordination system with a very low-density framework, and single crystal growth
and structure refinement of the titano-silicate ETS-10.
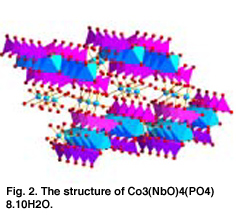 The synthesis and properties of oxides that have applications in high temperature
ionic devices, such as fuel cells, oxygen transport membranes and sensors is a second
research area. The major part of our work centers on mixed metal oxides with the ABO3 perovskite structure. Because the database of perovskite oxide ionic properties is
limited, we investigate new compositions to establish structure-property relationships.
We use a variety of techniques to characterize the surface reactivity and bulk transport
properties of materials. We use oxygen permeation through membranes, electrical conductivity
relaxation, dc conductivity, ac impedance spectroscopy and other techniques to establish
transport properties at high temperature in a variety of gas atmospheres. Isotope
exchange together with secondary ion mass spectroscopy depth profiling, gives important
and complementary information about ionic transport across interfaces.
The synthesis and properties of oxides that have applications in high temperature
ionic devices, such as fuel cells, oxygen transport membranes and sensors is a second
research area. The major part of our work centers on mixed metal oxides with the ABO3 perovskite structure. Because the database of perovskite oxide ionic properties is
limited, we investigate new compositions to establish structure-property relationships.
We use a variety of techniques to characterize the surface reactivity and bulk transport
properties of materials. We use oxygen permeation through membranes, electrical conductivity
relaxation, dc conductivity, ac impedance spectroscopy and other techniques to establish
transport properties at high temperature in a variety of gas atmospheres. Isotope
exchange together with secondary ion mass spectroscopy depth profiling, gives important
and complementary information about ionic transport across interfaces.
- US Editor, Solid State Ionics
- Associate Editor, Materials Research Bulletin
- Editorial Advisory Board, Progress in Solid State Chemistry, Journal of Solid State Chemistry, Solid State Sciences