New Sensors Can Reveal Therapeutic Targets, Impact of Probiotics
A University of Houston researcher is developing a new set of metal sensors that will be able to function in the gastrointestinal tract, a low-oxygen environment, to examine how gut bacteria respond when trace metal nutrients, like iron and zinc, are thrown out of balance either through diet or disease.

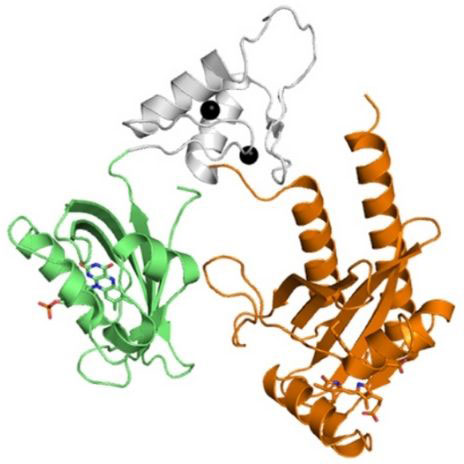
“We are developing new fluorescent metal sensors that do not rely on oxygen so that they can be applied to gut bacteria cultures under low oxygen or anaerobic conditions,” said Melissa Zastrow, assistant professor of chemistry. Zastrow has been awarded $1.9 million from the National Institute of General Medical Sciences to develop her protein-based metal sensors.
Trace metal nutrients are tightly regulated in living systems to avoid deficiency or toxic overload, but metal levels in the gastrointestinal tract vary with diet. Dietary metals affect the colonization of bacteria and the ability to resist the impact of infectious bacteria, leading to an increased chance of infection or gastrointestinal diseases.
But how that happens, the molecular mechanisms at play, remains largely unknown. Understanding how diet changes the gut microbiota and its function should lay the foundation for disease treatment and prevention.
“This lack of knowledge severely limits our ability to predict how diet or host metal status will impact treatment of gastrointestinal diseases or infection. Our long-term goal is to elucidate the molecular mechanisms governing how essential metals affect the human gut microbiota,” said Zastrow, a faculty member in UH’s College of Natural Sciences & Mathematics.
Researchers have been detecting metals in biological systems for years, typically with fluorescent sensors made from synthetic materials or green fluorescent proteins (GFP), which require oxygen to become fluorescent. Since a lot of gut bacteria cannot survive in the presence of oxygen and the gut is a mostly oxygen-free environment, GFP-based sensors do not work well for studying them.
Zastrow’s sensors will use proteins, which she prefers since they can be sent to a specific target, like a single type of bacterial species, but they won’t require oxygen to become fluorescent.
“Oxygen-insensitive protein-based fluorescent sensors will be used in live anaerobic cultures containing Lactobacillus species to study metal uptake and how metal ion levels vary over time,” said Zastrow, who will also examine how essential metals affect probiotic Lactobacillus species.
Probiotic bacteria, which deliver health benefits when consumed in adequate amounts, have long been used to enrich gut health. Despite decades of research, however, probiotic effectiveness is debatable and often conflicting, so there is significant need to understand molecular mechanisms underlying probiotic impacts and how these are affected by metals. Zastrow said that information can lead to better, individualized treatment.
“If you understand what makes up a patient’s gut community and how it is functioning, then you can potentially make more informed decisions about how to treat them,” said Zastrow.F
- Laurie Fickman, University Media Relations