Researchers from the University of Houston and Trinity University have for the first time provided direct evidence of a water-mediated reaction mechanism for the catalytic oxidation of carbon monoxide.
The work used gold nanoparticles and titanium dioxide as a catalyst to speed the process and determined that water serves as a co-catalyst for the reaction that transforms carbon monoxide into carbon dioxide. While researchers have worked with carbon monoxide oxidation using gold catalysts for years and have realized that water can change the reaction, none have previously been able to fully explain why it worked.
The work is described in the Sept. 5 edition of the journal Science and online at http://www.sciencemag.org/content/early/recent.
“We can say with a high degree of certainty that we now understand the role of each of the components and what they do during this catalytic reaction,” said Lars Grabow, assistant professor of chemical and biomolecular engineering at the University of Houston. He and Hieu Doan, a Ph.D. student at the UH Cullen College of Engineering, developed computational simulations to support experiments run by Trinity University chemists Bert Chandler, Christopher Pursell and Johnny Saavedra.
Chandler, professor of chemistry at Trinity, said the work was a true collaboration.
“It took all of us to make it happen,” he said. “What we did is to bridge the gap between surface science and computational people. We knew water helped the reaction but didn’t fully understand its role. Now we know that water is a co-catalyst for this reaction.”
When used in jewelry, gold is prized for its nonreactive properties – it doesn’t rust or tarnish when exposed to air or water. And researchers have long known that, despite its reputation as an inert metal, gold nanoparticles can work as a catalyst to speed chemical reaction.
But nobody knew exactly why it worked. Water turned out to be key, even when it isn’t explicitly added to the process, Grabow said.
Trace amounts of water drawn from the air drove the reactions on the surface of the gold catalysts, he said.
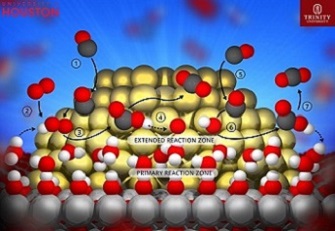
During the experiments and computational study, the researchers looked at how water, surface hydroxyls and the metal-support interface interacted during carbon monoxide oxidation over a gold-titania catalyst.
“In all cases, an essentially barrier-free proton transfer lowered the overall energy of the system, generating H2O2 or OOH. Once OOH formed, it migrated along the Au particle, allowing atoms near, but not strictly at, the metal-support interface to participate in the reaction,” they wrote to describe their findings, referring to the generation of hydrogen peroxide or hydroperoxyl and hydroperoxyl’s migration along the gold particles.
Essentially, they found that protons from a thin layer of water stretching across the surface of the catalyst detach from the water molecules and attach to oxygen molecules, briefly moving onto the surface of the catalyst to spur the reaction before returning to the water layer.
Previous models typically focused on individual components of the reaction, Grabow said, making this project the first to pull all of the facets together in a single model that fully supports the Trinity chemists’ experimental observations.
Chandler said the work could provide a way to produce clean hydrogen from petroleum and natural gas.