
A Hypothetical Situation
Igneous rocks on the planet Erehwon contain the minerals redite. blueite, and yellowite. Consider a melt with:
redite - 9 parts
Different magmas may contain a different number of parts and, in order to compare them, one can convert these data to percentages - a percentage is the number of parts per 100 parts. Therefore, if there are 9 red parts per 36 total parts (the sum of all of the parts) there are: (9/36)*100 or
25% redite
A proportion is really parts per 1 part. There are 0.25 parts of redite per 1 part of magma in this example.
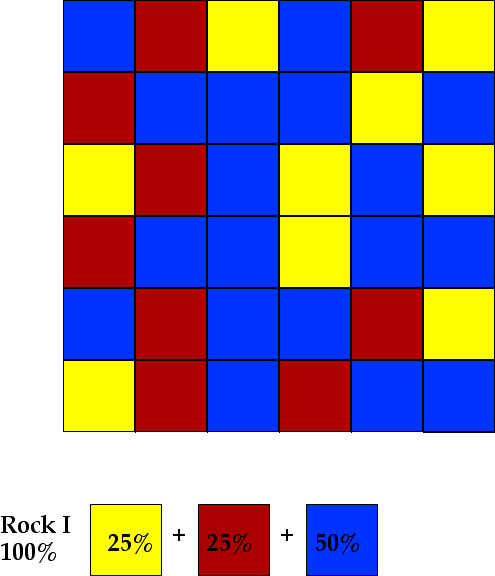
If nothing is added to or subtracted from this magma it will crystallize to give a rock with a mineral composition identical to that of the magma. If nothing is added to or subtracted from a granitic magma, a granite will form when the magma is slowly cooled. We will assume that blueite crystallizes first (at the highest temperature), yellowite crystallizes second and redite crystallizes third. There is some overlap so that some blueite and yellowite and yellowite and redite crystallize simultaneously. This situation will be modeled as illustrated below. There are 36 total parts (squares) with 9 parts redite, 9 parts yellowite and 18 parts blueite.
yellowite - 9 parts
blueite - 18 parts
Percentages are an interesting numerical transformation. Percentages preserve the ratios of variables. Note that (parts A/parts B) = 1 and (%A/%B) = 1. Convince yourself that all ratios are preserved by the percentages. Note that the sum of the percentages of all of the parts must be 100% -- some of it plus the rest of it equals all of it!
(parts/total parts)*100
25% yellowite
50% blueiteEquilibrium Crystallization

A single rock forms (Rock I) with percentages as shown in the figure.
Fractional crystallization refers to processes which separate crystals from liquid. When this happens the liquid that remains is considered as a new magma. A number of processes can cause the crystals to be separated from a magma. Many minerals are denser than the liquid that they crystalize from. For example, olivine has a density of about 3.2 g/cc as compared to about 3.1 g/cc for a basaltic magma. In the absence of convection currents that would tend to keep the crystals suspended within the liquid, the denser crystals would sink to the bottom of the magma chamber. Similarly, crystals that are less dense than the liquid would tend to rise to the top of the magma chamber. [Based on your previous work, what mineral(s) would you expect to be form from a basaltic melt and be less dense than the melt?]. When the separation occurs, the previously formed crystals are no longer able to react with the liquid.
Consider a case in which 9 parts of blueite are separated from the liquid. The remaining liquid must contain 9 parts of each of the three minerals - there are still 36 parts total. This situation is shown below.
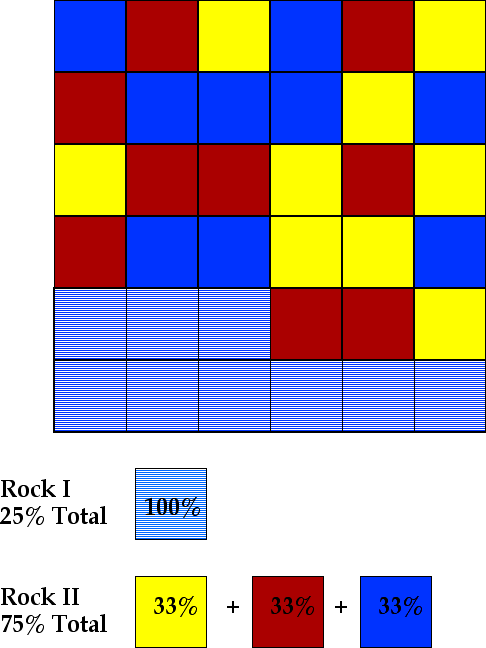
Note that two rocks (sets of mineral assemblages) are formed. Rock I consists of 9 units of the early separated crystals of blueiote which are shown with a lined pattern. Rock II consists of 9 parts of blueite, 9 parts of redite, and 9 parts of yellowite.
Rock I is equivalent to 25% of the starting magma - 9 parts of B out of 36 total Parts - and Rock II is equivalent to 75% of the starting magma. Note that the bulk composition (both rocks considered together) is unchanged - 18 parts blueite, 9 parts redite and 9 parts yellowite : each mineral occupies 33% of Rock II.. The distribution, has been changed by the removal of the early formed crystals of blue. Note that the percentages of yellowite and redite are 33% each. Fractional crystallization has produced a rock with an enrichment of these two components. Also note, that the total amount of redite and yellowite remains the same for the total system -- each is still 9 units.
Thus, crystall fractionation increases the diversity of the rocks that can be formed by the crystallization of a magma.
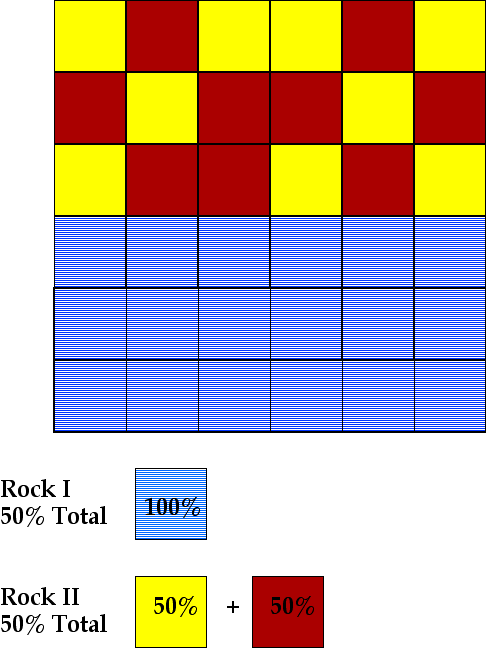
A second scenario results in all of the crystals of blueite being separated from the magma (the lined pattern). The remaining magma (50% of the starting magma) crystallizes a rock with equal amounts of redite and yellowite. Again, note that the bulk composition is unchanged when both sets of mineral assemblages (Rock I and Rock II) are considered.
Crystal fraction has produced a rock (Rock II which has 50% of redite, the mineral which crystallizes last in the sequence -- twice the percentage of redite in the assemblage that forms without crystal fractionation.
Thus, crystal fractionation acts as a mechanism for enrichment. Imagine that redite has a commerical value if its concentration exceeds 40%. . Without crystal fractionation, the magma yields a rock with 25% redite and it would cost more to remove red than you would gain by selling it. In the first scenario, crystal fractionation produced one rock with 33% redite which is an enrichment but still insufficient to recover redite and make a profit. The third scenario, however, produces a rock with 50% redite which, in this hypothetical situation, could be recoved at a profit.
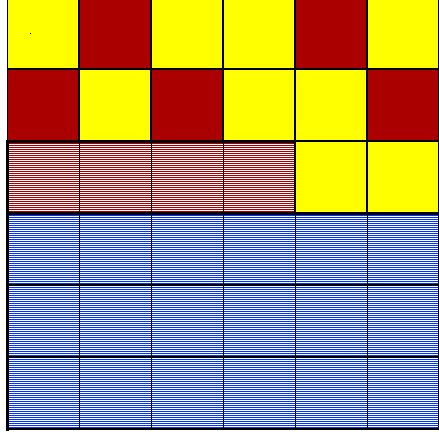
You task is to evaluate a scenario in which 18 parts of bluite and 4 parts of redite (both shown below with a lined pattern) are separated from the magma to form Rock I. This leaves 5 parts of redite and 9 parts of yellowite in the remaining magma. This crystallizes and forms Rock II.
Test your understanding of crystal fractionation by taking the self-evaluation that follows.
Return to the Physical Geology Home Page