
In general, geologic processes do not operate in isolation. Changing one condition in one cycle may result in a change in other processes operating in other cycles. For example, the subduction of marine limestones and the eventual melting releases carbon dioxide into the atmosphere during volcanic eruptions. Increased carbon dioxide will increase the temperature of the air and the Earth's surface.
In the text Lovelock's Daisyworld model was introduced; recall that only Black and White daisies inhabit this planet. When the temperature rises the Black daises die because they absorb heat whereas the White daisies reflect heat into the atmosphere. As the proportion of White daisies increases, the temperature decreases and the Black daisies increase in abundance. Notice that there is a negative feedback in the sense that changing one thing (temperature) results in a change in another (the proportion of White daisies) which acts to bring the system back to where it started. Some systems exhibit a positive feedback mechanism which causes the system to rapidly move away from where it started; for example, the increasing frequency of a sound system which is not properly tuned (hence the name "feedback"). A company makes money, invests in new machinery, produces more of its product and makes even more money. This would create an upward spiral. Sometimes the spiral can be downwards....sort of like "its just one damn thing after another...."
In chemical reactions the principle of Lechatelier is often used to make predictions to the "what if question" - if a system is at equilibrium and a disturbance occurs, the system changes to undo the effect of the disturbance. Consider the following chemical system:
If the amount of the Ca ion is increased the system should shift to the left to reduce the Ca concentration; that is the system will shift to the left and CaCO3 will form. If the Ca ion were reduced the system would shift to the right and calcite would break down.
There are a number of measures that could be used to assess the "health of a society". The following diagram illustrates the variation of three key measures: Gross Domestic Product, Population and Energy Consumption.
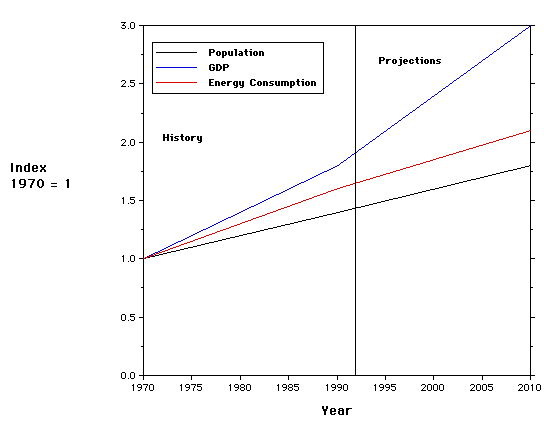
The plot illustrates a useful way to display different types of information on the same graph. The 1970 values of population, energy consumption and the gross domestic product are used as the denominators in forming three ratios which are plotted versus time. For example, the population in 1993 is divided by the population in 1970 - ~1.4. Such a ratio is called an index. Questions - what is the significance of an index that is less than 1.0? From the graph, compare the doubling rate of population and energy consumption. Is there cause for alarm?
A good place to begin is to look at the balance between energy production and consumption for the United States. Note that in 1994 the U.S. consumed some 18 quadrillion Btu more than it produced. Projected energy needs and estimates of how long our known energy resources will last should be sobering. Many of the decisions that you will help make will focus on the wise use of known energy resources and potential investments in alternative sources of energy.
Earth System Science has become a popular "buzz phrase" during the past few years and many universities (including UH) are offering courses which emphasize the systems approach to geology. Florida State is offering such a course and the following link will let you read through a short essay illustrating the major Earth systems: FSU.
A trip to the Summitville Mine will introduce you to the relationships between mining and the environment.
Or, try the following as an example of the relationships between geologic processes and the use of the environment.Geologic Features at a Sludge Dump Site
Recall that approximately 97% of the weight of the Earth's crust is contributed by eight elements - oxygen, silicon, aluminum, iron, sodium, calcium, potassium and magnesium. This means that elements of value to our society (such as chromium, tin, copper, and gold) account for less than 3% of the weight of the crust. If these elements were homogeneously distributed (the same concentration throughout the crust) the cost to extract many of them would be prohibitive. An ore is a deposit from which an Earth resource is economically recoverable; that is, a profit can be made. As technologies and demand change, a deposit once judged to have no economic value may become an ore. Extraction of an ore may creates a land use problem as evidenced by this large open pit copper mine near Clifton, Arizona.
Titanium, for example, makes up about 0.60% by weight of the crust. That is, 100 grams of average crust would contain about 0.60 grams of titanium. If it takes about 30% titanium to constitute an ore (30 grams per 100 grams), a concentration factor of about 50 is required. Fortunately, there are many processes which lead to natural concentrations of the elements (minerals) that make up the crust.
Review Bowen's Reaction Series from the Chapter on Igneous Rocks. Olivine, a magnesium/iron silicate, crystallizes at high temperatures from a magma. Other elements will distribute themselves into the solid or the liquid. [The partition coefficient is the ratio of the amount of an element in the solid to the amount of the element in the liquid.] Chromium, for example, will substitute for the iron and magnesium preferentially and its distribution coefficient is a large number. If 0.1% chromium is present in the magma (1 part in 1,000 parts) and if olivine represents 10% of the crystallized magma, then essentially all of the chromium is concentrated in 10% of the crystallized melt. This amounts to an enrichment factor of about 10.
Question If olivine formed 5% of the crystallized melt what would be the enrichment factor for chromium be? If the olivine settled out of the magma chamber (due to its relatively high density) then one might find a valuable ore of chromium associated with the base of thick gabbro sills.
Other elements, such as gold, uranium, silver, lead and copper, tend to become enriched in the melt. As crystallization proceeds, these elements, along with water and sulfur, usually increase in abundance in the remaining liquid. If these hot water (hydrothermal) solutions leave the magma chamber and penetrate the county rock they will eventually cool as they migrate upwards. Various sulfides or oxides may crystallize if the composition is correct. Gold, for example, usually does not form compounds and is precipitated at relatively high temperatures. Lead and zinc sulfides, however, do not precipitate until the temperatures are quite low. Thus, there may be a zonation of mineral deposits around an igneous pluton.
Hydrothermal fluids are also associated with some spreading centers. Hot sea water can circulate through hot basalts, dissolving some elements and redepositing them under cooler conditions. Black Smokers provide a fascinating look at the biological, chemical and physical processes associated with some active spreading centers.
50% of volume remaining: calcium carbonate (calcite) [2.8g/cc]
20% of volume remaining: calcium sulfate (anhydrite or gypsum) [2.4g/cc]
10% of volume remaining: sodium chloride (halite) [2.1g/cc]
Thus, a layered deposit with calcite at the base, then anhydrite or gypsum and halite at the top could form in a closed basin. If the process was repeated (by the periodic freshening of the body of water) layers of salt (with its low density) become covered by more dense material creating an inverted density stratification. At high temperatures and pressures halite behaves plastically and salt domes can form. The opening of a "new" ocean basin often has provided an ideal environment for the deposition of thick layers of halite. Halite has a very low permeability and salt domes often serve as a trapping mechanism for petroleum.
| jbutler@uh.edu
|ClassListserv
|Textbook Home Page
|Glossary of Geologic Terms|
|Search These Pages|Other Courses|Resources|Grade Book|
Copyright by John C. Butler, July 29, 1995