Treat the following as an electronic text. Some of the following has been covered in the lecture material but go through the entire page. Do the quizzes. There is nothing to be handed in from this Internet-distributed exercise. A problem will be distributed in class and it is due on the date given in the reading list.
Introduction
A rock sample contains calcite, quartz and wollastonite. Does this assemblage represent attainment of equilibrium under some set of conditions attained in the past? What is meant by equilibrium? If the assemblage is not an equilibrium assemblage how long would it take to reach equilibrium? Given the bulk composition represented by the sample, what is the equilibrium composition at 1000o C? How does this assemblage change as the temperature changes?
To begin to answer these types of questions we need to become familiar with some fundamentals of thermodynamics and kinetics. Thermodynamics evolved from a number of observations that mechanical energy is transformable into heat. The underlying principles lead to a formal statement of chemical equilibrium which will be helpful in thinking about naturally occurring systems.
Kinetics deals with rates and mechanisms of reactions. For example, thermodynamics will tell us that carbon in the form of graphite is stable with respect to carbon in the form of diamond at 25o C and 1 atmosphere total pressure. If this "law of thermodynamics" was instantaneously "obeyed" there would be a lot of upset individuals who have invested heavily in diamond jewelry. Kinetics will help us understand the structural changes that must take place to convert diamond into graphite and the rate(s) of the reaction(s).
A system is a part of the universe which an investigator has defined and whose properties are of interest. Everything else is the surroundings. Note that the specification of the system is done by the investigator. The system could be a rock unit, a hand sample, or the chemical system:
C graphite = C diamond (1)
A boundary separates the system from the surroundings and there are several kinds of boundaries. In an open system matter can pass across the boundary whereas in a closed system matter is prevented from entering or leaving the system. Boundaries which will not allow heat or other forms of energy to pass through create isolated systems.
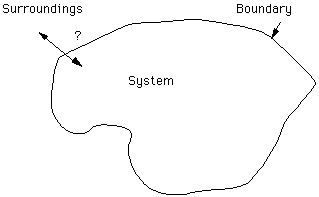
The properties of a system are either intensive or extensive. Intensive properties, such as temperature, pressure and density, are independent of the amount of matter. Extensive properties such as volume depend on the mass and are additive.
The chemical composition of a system is expressed in terms of phases and components. A phase is a uniform, homogeneous, physically distinct, and separable part of a system. For example, H2O exists in three phases (at low T and P) - ice, water, and steam. The minerals calcite, quartz and wollastonite in the rock noted previously are three phases. The components of a system are the chemical constituents which are used to describe the compositions of the phases in the system. The designation of the components is arbitrary. For the system H2O there is one component - H2O. For the system containing calcite, quartz, and wollastonite the components could be SiO2 - CaO - CO2. The composition of all three phases can be represented by a combination of one or more of these components. Wollastonite, for example can be represented as
CaSiO3 = CaO + SiO2. Systems with one component are unary systems...two are binary systems ... three are ternary systems.
The phase rule: p + f = c +2 - relates the number of phases (p), the number of components (c) and the number of degrees of freedom (f). In general the number of degrees of freedom is the number of variables (temperature, pressure, etc.) that must be specified in order to define the state of the system. In the section on phase equilibrium examples will be given to clarify the phase rule and its application.
In this introduction a qualitative approach will be taken with the emphasis on words rather than mathematics. The reader is cautioned, however, that this approach will not lead to a thorough understanding of the topics that are covered.
We will return to the system:
CaCO3 + SiO2 = CaSiO3 + CO2 (2)
Reactants Products
Forward => <=Reverse
Note that the system could be written as:
CaSiO3 + CO2 = CaO + SiO2 (2a)
which reverses the sense of products and reactants. The products are always on the right hand side of the expression and the reactants on the left.
Early on, chemists argued that a reaction proceeded in the forward direction at a rate R1 and in the reverse direction at a rate R2. At equilibrium R1 = R2. Equilibrium is dynamic. Once established, changes in either the products or the reactants or both can cause a shift which returns the system to equilibrium. LeChatelier's Rule states that when a system at equilibrium is disturbed the system will respond by undoinging the effect of the disturbance. For example, if CO2 is increased the system will shift to the left so as to use up the Ïexcess• in the production of calcite and quartz. If there is too much CO2 so that all of the wollastonite is consumed then the system as defined no longer exists.
Consider the following system:
Ba+2 + SrSO4 = Sr+2+ BaSO4 (3)
where Ba and Sr are ions in solution and celestite and barite are solids. The equilibrium constant (K) is defined as the ratio of the product of the activities of the components in the products to the product of the activities of the components in the reactants:
K = [(aSr+2)(aBaSO4)]/[(aBa+2)(aSrSO4)] (3a)
At this level we will consider that the activities of ions in solutions are equal to their chemical concentration in the solution. By definition the activity of a pure solid or a pure liquid is equal to 1.0. Therefore, for system (2) K is equal to:
K = [(Sr+2)/(Ba+2)] (3b)
At equilibrium the ratio of the concentrations of the two ions is a constant. For example, if at 25oC the equilibrium amounts of Sr+2 and Ba+2 are 100 ppm and 1 ppm respectively the value of the constant is 100. Departures of this ratio from this value (at 25oC ) are indications that equilibrium has not been attained. The value of this constant is usually very sensitive to temperature changes and less sensitive to pressure changes. For system (2) the equilibrium constant is given by:
K = (aCO2) (4)
If CO2 is an ideal gas then its activity is estimated by its partial pressure. That is, at equilibrium quartz, calcite and wollastonite are in equilibrium at a specific partial pressure of CO2. For example, if the equilibrium partial pressure of CO2 was 10-3.0 atm (at 25oC) and the system is exposed to atmospheric CO2 (10-3.5 atm) which way will the reaction shift? [Is the atmospheric CO2 partial pressure greater or less than the equilibrium partial pressure?]
Write the expression for equilibrium constant for the system:
CaCO3 = CaCO3 (5)
calcite aragonite
K = 1.
For systems that contain pure solids and liquids the equilibrium constant does not convey useful information.
Another way to analyze a chemical system is to compute the molar volumes - the volumes represented by the products and reactants. Recall that:
density = mass/volume.
The gram formula weights of calcite and aragonite are identical (100 grams per formula weight) and the densities of calcite and aragonite are about 2.7 and 2.9 respectively; therefore the volumes are 36.9 cc and 34.5 cc (where density is in g/cc).
CaCO3 = CaCO3 (5a)
calcite aragonite
36.9 34.5
Which side of the reaction will be favored at high temperatures?
Increasing pressure favors the side of the reaction (forward or reverse) with the smallest molar volume. Therefore, aragonite is a high pressure phase of calcite. Temperature most often has the opposite effect. Heating aragonite favors the formation of calcite (as long as the decomposition temperature is not reached). That is, pressure favors the side of the reaction with the highest density or smallest volume. As volume is an extensive property the volume occupied by a reaction in which there are several products (and reactants) is obtained by adding the volumes occupied by each of the products (reactants) together.
Temperature and Pressure Variations
During this course you will need to have a general model in mind for the variation of temperature and pressure with increasing depth.
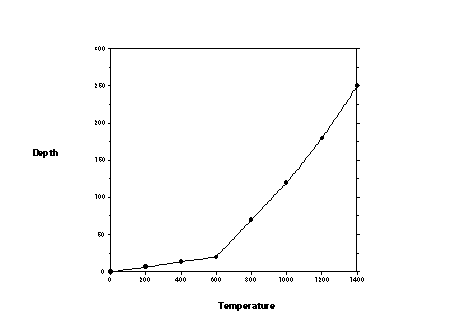
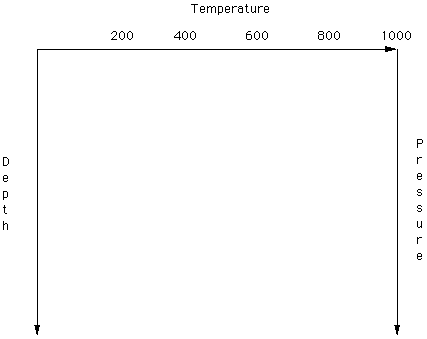
Heat flow measurements made in mines and drill holes reveal that temperature in the Earth increases (near the surface) at a rate of about 30oC per kilometer. [Convert this to oC per mile]. If the radius of the Earth is about 6,000 km, predict the temperature at this depth. No known solid or liquid materials can withstand this high a temperature so the rate of change of temperature with increasing depth is not a constant. A crude plot of the continental geothermal gradient is given in Figure 2. Normally, the gradient is drawn to that depth increases downward. On the blank graph in Figure 3 imagin sketching the geothermal gradient depicted in Figure 2.
When rocks are buried they are subjected to increasingly greater lithostatic pressure, which results from the weight of the overlying rocks. This pressure is applied equally in all directions. In addition to the lithostatic pressure resulting from burial, rocks may be subjected to differential pressures. Under these conditions the rocks will be distorted. A third type of pressure is that associated with the partial pressure of various gasses which may be present in the defined system.
In continental lithosphere the lithostatic (or load) pressure increases at a rate of about 0.33 kilobars per kilometer. This value reflects an average density of about 2.65 g/cc of continental rocks. For basaltic rocks or mantle rocks the rate of increase will be greater due to the greater density of the overlying material.
One kilobar (kb) = 1,000 bars = 1,000 atmospheres = 14,700 lbs/in2.
On Figure 2 convert depth to Load Pressure (continental lithosphere) and reproduce on Figure 3. (You may wish to print this figure. Point at the image on the screen (with your mouse) and hold down on the "clicker". Select Open This Image and then Print (under the File heading).
Thermodynamics
Chemists noted that most reactions are accompanied by a loss or gain in temperature or a change in the heat content. Changes in the heat content (changes in the enthalpy of a reaction) are referred to as DH. If the system looses heat to the surroundings the reaction is defined as exothermic. By convention, exothermic reactions have a negative D H. If the system takes in heat from the surroundings the reaction is said to be endothermic and DH is +. There are tabulated values of quantities defined as the changes in the enthalpy of formation for ions and compounds. For the system:
CaCO3 = CaCO3 (5b)
calcite aragonite
-288.45 Kcal -288.49 Kcal
(kilo calories - a calorie is the amount of heat needed to raise the temperature of one gram of water 1 o C. Changes in enthalpy (and other thermodynamic quantities) are given by subtracting the sum of the enthalpies (or other quantities) of formation of the reactants from the sum of the enthalpies (or othre quantities) of formation of the products. Each value is multiplied by the number of formula units of the particular phase in the chemical system. Enthalpies of formation for calcite and aragonite are given above:
-D H = (-288.49)-(-288.45)
= -0.040 Kcal or - 40 calories.
Therefore, the forward direction (calcite to aragonite) is:
and the reverse direction (aragonite to calcite is the opposite). Rewrite this system as:
CaCO3 = CaCO3
aragonite calcite
Note that D H is opposite in sign but equal in absolute value to that for the system as previously written.
CaCO3 = CaCO3 (5c)
calcite aragonite
=> exothermic
<= endothermic
If this system is at equilibrium and the temperature is increased what will happen? The system will shift so as to favor the side that takes in heat (reduces the temperature):
If heated at 1 atmosphere, aragonite will be converted to calcite (assuming that the decomposition temperature is not reached).
Many, but not all, exothermic reactions are spontaneous. Consider the following system:
H2O = H2O (6)
water gas
-68.3 Kcal -57.8 Kcal
From the enthalpies given above determine whether the forward direction is endothermic or exothermic.
Interpret this result based on your experience with heating water.
As part of an attempt to be able to predict the direction of spontaneous change, chemists defined another quantity - Entropy (S) - as a measure of the degree of order or disorder in a chemical system. Crystalline solids are highly ordered as the constituents (atoms or ions or molecules) occupy regular positions in the structure. Gases, on the other hand are highly disordered as the molecules in a gas are in constant motion at all temperatures above absolute zero. Liquids have a degree of order intermediate between solids and liquids. Amorphous solids (glass, opal, etc.) have structures resembling those of liquids and have intermediate order.
H2O = H2O (6a)
water gas
16.7 cal 45.1 cal
Generally, the values of entropy (as given above) are several orders of magnitude smaller than those of enthalpy. The change in entropy of a reaction (DS) is given (for the above system) by:
DS = 45.1 - 16.7
= 28.4 cal
If the change in enthalpy is positive the products are more disordered than the reactants. Thus, steam is more disordered than water. Many, but not all, spontaneous reactions favor the disordered side of a chemical system.
Neither enthalpy or entropy is individually capable of predicting the direction of spontaneous change. The change in Gibbs Free Energy of a reaction is defined as: DF = DH - TDS (7)
where T is the temperature in degrees Kelvin. If:
D F = - products are stable with respect to the reactants
D F = + reactants are stable with respect to the products
D F = 0 system is at equilibrium
CaCO3 = CaCO3
calcite aragonite
-269.78 Kcal -269.53 Kcal
D F = -269.53 - (-269.78) = + 0.25 Kcal
What is the direction of spontaneous change for this system?
Therefore, calcite (the reactant) is stable with respect to aragonite (the product) at 25 oC. We know, however, that aragonite can be formed at 25 oC by some clams and that it persists for a relatively long time. A careful analysis of carbonates in the rock record reveals that the amount of aragonite decreases with increasing geologic time. Phases which form and persist for a finite time period in spite of not being predicted from thermodynamics are termed metastable. An explanation of why aragonite forms and persists requires the application of chemical kinetics. It is important to be careful how you describe a system. The statement that calcite is stable with respect to aragonite at25 oC is correct. However, the statement that calcite is stable at25 oC is not as there may be some systems in which calcite is not part of a spontaneously formed assemblage.
Using the expression D F = D H - T DS and the definition of equilibrium (F =0) derive an expression that allows you to estimate the equilibrium temperature (in degrees Kelvin) for a reaction.
Tequilibrium=DH/DS is the correct expression.
What assumptions must you make in order to make this calculation? [The ratio of DH toDS does not change from 25oC to the equilibrium temperature]
For the system:
H2O = H2O (8)
water gas
You have already computed
D H = +10.5 kcal and D S = 28.4 kcal. Making sure that both variables
have the same units, estimate the equilibrium temperature for the system.
Follow through the following analysis to test your understanding of some
of the topics that have been presented in this exercise.
D F = D H - T D S :
The forward direction will be the direction of spontaneous change if,
and only if, D F is negative. For any given system there are a limited
number of possibilities:
_______________________________________________
D H D S Forward is spontaneous
_______________________________________________
1. - + always
2 - - __________________
3 + + __________________
4 + - never
Thus, if the forward direction is exothermic and in the direction of
increasing disorder (1), the forward direction is also the direction of
spontaneous change as DF will always be negative.
If the forward direction is endothermic and in the direction of increasing order (4),
the forward direction will never be the direction of spontaneous change
as D F will always be positive.
If the forward direction is exothermic but in the direction of
increasing order (2), the forward direction is the direction of
spontaneous change unless the quantity (TDS) is > DH.
Be prepared to analyze case (3) above.
The Relationship Between K and DF
It should seem logical that there is a relationship between
the equilibrium constant K and DF as both describe a system at equilibrium.
The relationship is:
log(K) = -(DF/1.364) (9)
That is, the change in the free energy of the reaction can be used
to compute the log of the equilibrium constant (or vice versa).
For the system:
FeO + CO2 = FeCO3 (10)
DF = (-161.06)-(-58.4)-(-94.26) = -8.40 Kcal
the equilibrium constant is:
K = 1/p CO2
Therefore,
log(K) = -(-8.40)/1.364
= +6.1 and
pCO2 = 10-6.1 atm
Recall that the partial pressure of pCO2 is 10-3.5 atm which is much greater than the equilibrium partial pressure for this system.
This relationship can be used to determine a numerical evaluation of the equilibrium from the change in the free energy of the reaction. The user must interpret this value with respect to the equilibrium constant formulation. In system (3) the equilibrium constant was written as the ratio of the concentrations of two ions: K = [(Sr+2)/(Ba+2)]. The numerical evaluation gives the ratio of the two ions at equilibrium.
If Keq for this system is 105 then:
If system (3) is at equilibrium and brought into contact with water in which Sr+2
is twice as great as Ba+2, the system will react by:
favoring the forward direction
favoring the reverse direction
The following analysis is taken with some modification from Solutions, Minerals, and Equilibria by Garrels and Christ, 1965 which remains one of the most comprehensive introductions to the application of chemical thermodynamics to the solution of geological problems.
Consider the system:
Fe2O3 + 3H2O = 2Fe3+ + 6OH- (11)
(2 x - 2.53) + (6 x -37.6) -(-177.1)-(3 x -56.69)
From tabulated values of changes in the free energy of reaction, D F = +116.51 kcal so that the reactants are the direction of spontaneous and are stable with respect to the products. Note that the free energy of formation of water is multiplied by 3 because there are 3 formula units of water present in the system.
The equilibrium constant is given by:
K = [Fe3+]2[OH-]6/[Fe2O3][H2O3]
Since water and hematite are pure, there activities (or chemical concentrations) are equal to 1.0 so that:
K = [Fe3+]2[OH-]6
From (9),
log(K) = -(116.51)/1.364
= -85.4 = log( [Fe3+]2[OH-]6) or
-85.4 = 2log[Fe3+] +6log[OH-]
2log[Fe3+] + 6log[OH-] = -85.4 or
log[Fe3+]+ 3log[OH-]= -42.7
This is the equation of a straight line on a graph where the axes of reference are log[Fe3+] and log[OH-] . The resulting plot can be labeled to indicate the conditions where the reactants are stable and where the products are stable. The straight line represents what is required (in terms of the concentrations of these ions) to attain equilibrium.
For solutions, the common practice is to cast the system with respect to H+ rather than OH-. This can be accomplished by adding the equation for the dissociation of water to the system equation:
Fe2O3 + 3H2O = 2Fe3+ + 6OH-
6OH- + 6H+ = 6H2O
_____________________________
Fe2O3 + 6H+ = H2O+ 2Fe3+
log K = log(Fe3+)2/(H+)6
or, log K = 2log(Fe3+) - 6log(H+)
by definition, pH = -log(H+) so
log K = 2log(Fe3+) + 6pH
Note that each unit step in pH corresponds to an order of magnitude step in H+. For example, a solution of a pH of 5 has 10-5 moles per liter of the hydrogen ion.
What is the pH of a solution with 10-10 moles per liter of the hydrogen ion?
What is the concentration of hydrogen ions in a solution with a pH of -2?
