Cancer Treatment of Tomorrow

A genome sequencing pioneer in the field of microRNA research and application, Gunaratne sees tailor-made cancer treatments in the near future. An ardent supporter of personalized medicine, she is particularly excited about the $215 million Precision Medicine Initiative, announced by President Barack Obama, which is designed to leverage personal characteristics to develop more effective cancer treatments. Gunaratne says it validates the increasing need for personalized approaches in the health care industry.
“Ultimately, we anticipate that all patients who present with cancer will have their tumors subjected to whole genome sequencing,” she says. “This will give us a full view of all the things that have gone wrong. This genomic portrait of each patient’s tumor will then be used as a framework for practicing precision medicine.”
Gunaratne was a key contributor to The Cancer Genome Atlas (TCGA), a flagship project of the National Cancer Institute and the National Human Genome Research Institute of the National Institutes of Health, established to leverage advances in genome sequencing technologies. The TCGA consortium of scientists were tasked with the ambitious goal of finding every single genetic change associated with 20 cancers by sequencing tumors from 500 patients for each cancer. This effort has yielded an unprecedented view of the cancer genome.
Gunaratne says the advances in genome sequencing technologies are unraveling the genetic blueprints underlying cancer and other complex diseases. Specifically, her contributions to the TCGA consortium included elucidating the role of a tiny class of noncoding genetic molecules called microRNAs and pinpointing those that could unleash the body’s natural cancer-fighting agents.
While attention has long centered on DNA, little consideration has been given to its cousin RNA or to microRNAs, which were once considered “genetic junk” that played no significant role in the human genome due to their size and inability to make proteins. In the last decade, though, these once-dismissed tiny molecules have catapulted to occupy a central position in biology.
“DNA are certainly the building blocks that govern many of our processes, but only about 3 percent of the genome codes for protein,” Gunaratne says. “If we are to truly take the precision medicine effort all the way, we must also pay attention to the 97 percent of our genome that is noncoding that was previously considered trash, but now emerging as containing quite a bit of functionality.”
She goes on to say that while researchers understand the contribution of protein coding, work must be done to decipher what role the noncoding part of the genome plays in cancer and other diseases. She adds that the TCGA also revealed that only 10 percent of the genome is druggable using conventional drugs and methods. She says that’s only about 350 of a person’s 30,000 genes.
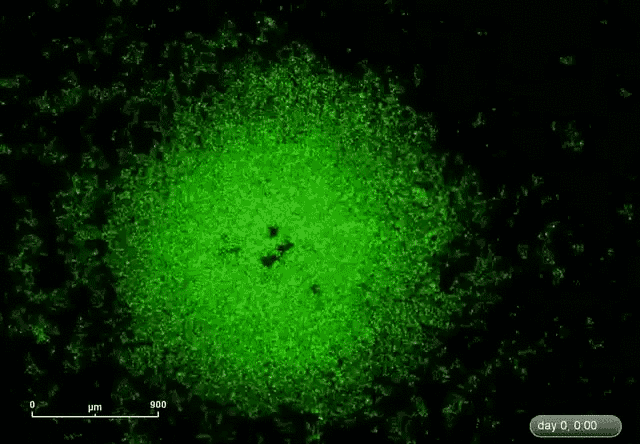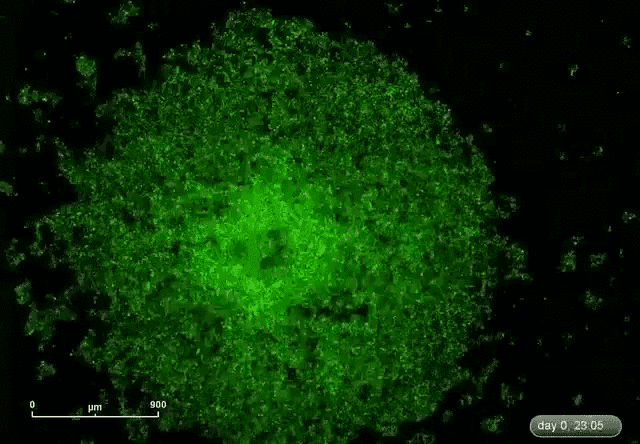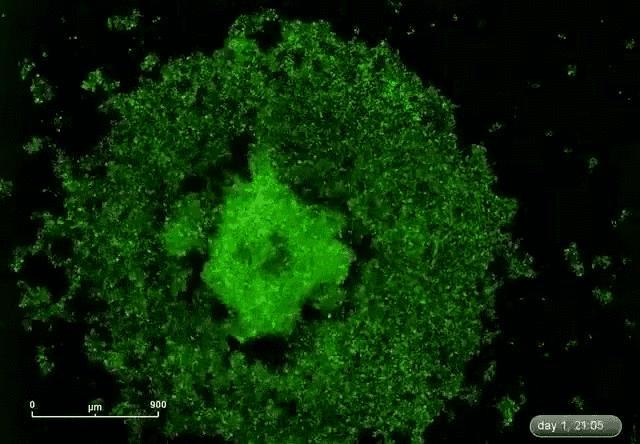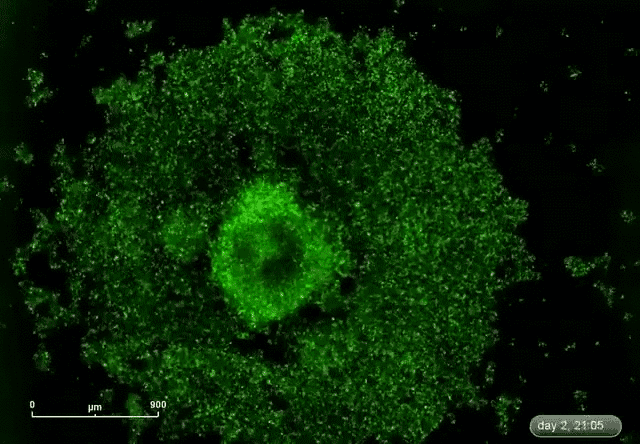
This is where microRNA comes in. Gunaratne says researchers have discovered about 2,000 microRNAs that can target and silence 80 percent of the 30,000 genes. This expands the druggable genome from 10 to 80 percent.
“Our plan is to use this expansion of druggability to develop the next-generation cancer drug,” Gunaratne says. “In the course of our research, we learned that a single microRNA can target and silence hundreds of genes. They are the circuit breakers we use on a day-to-day basis in responding to various outside insults, preventing disease. This provides us with a beautiful scheme where we can very quickly respond to a large number of mutations. If we want to take a whole network of cancer-causing genes out, we deploy a microRNA circuit breaker. The implications of this in developing future drugs are huge.”
She offers a compelling analogy, likening microRNA to a drone.
“If you think about it, the analogy is that cancer is a terrorist hiding in our body, and chemo is like bombing the village and hoping it finds the terrorist,” Gunaratne says. “If we use microRNA to narrow down the target, it’s like having a little delivery truck with the exact address of where the terrorist lives, and we deliver the bomb to the house.”
Gunaratne has chosen to target ovarian cancer, because it’s the most lethal gynecological malignancy, but her group has identified a unique microRNA that has the potential to be effective against 80 percent of cancers beyond just ovarian. She and her team have developed three lead candidate microRNAs that activate a specific protein.
“About 80 percent of cancers have a defect in the p53 protein, which is the guardian of our genome, so we know cancer cells are accustomed to and adept at outmaneuvering this protein,” Gunaratne explains. “Our microRNA lead candidate, however, activates the p63 protein, an ancient family member from which p53 evolves.”
Used for development, p63 is not generally present in high levels once we’re fully developed, since it’s no longer needed. As an ancient relative to p53, however, p63 can perform many of the same functions, yet is rarely mutated by cancer. By awakening p63 with their lead candidate microRNA, the researchers hope to circumvent mutations in p53 by launching a surprise attack on cancer cells.
“When we started, microRNAs were very outside the box, but now there’s buy-in from the community that these could be valuable therapeutic agents, and the next step is to move toward clinical trials,” Gunaratne says. “I think we are very well-positioned to be the first microRNA to be tumor targeted, as well as the first microRNA to circumvent mutations in p53 by activating p63.”
Responding to a CPRIT new company formation grant, Gunaratne’s group established Next microRNA Technologies. Preclinical work has shown their lead candidate microRNAs are able to both destroy cancer cells and sensitize tumors to chemotherapy. Their plan in establishing this company is to complete the preclinical work and then go to Phase 1 clinical trials.
“CPRIT is especially supportive of out-of-the-box type work and was the first to enable me to start this research,” Gunaratne recalls. “I don’t think these efforts would ever be funded if not for CPRIT.”
Gunaratne and her team also have partnered with SynerGene Therapeutics, a biopharmaceutical company that develops targeted diagnostics and therapeutics. SynerGene already has had success with a delivery platform technology that penetrates tumors and delivers therapeutics specifically into cancer cells. This intravenously administered, tumor-targeting nanoparticle has safely delivered the human tumor suppressor gene p53 in Phase 1 and 2 clinical trials. Once Gunaratne’s team gets approval for their payload of microRNAs to be carried in this delivery vehicle, their goal is to be the first microRNA in clinic that’s tumor targeted.
“We have to bring the noncoding genome in,” asserts Gunaratne. “It’s not like chemo will be excluded. It’s a matter of how we can all come together and how we can tailor make it to a patient’s tumor.”
Next Story:
Global Groundbreaking Discoveries
UH Center uses laser mapping around the world to produce valuable research data …