INTERORGAN
METABOLIC PATHWAYS
How does the various organs interact and what is the flow of
metabolites among them?
Cori Cycle (Fig. 21-6)
·
In
Muscle:
o
Fast-twitch
muscle metabolizes glucose to lactate
for formation of ATP.
o
Slow-twitch muscle usually
generate energy by Oxi Pho but also produces
lactate when ATP demand exceeds oxidative flux.
·
Lactate
is transported from muscle to liver via bloodstream.
·
Muscle
does not have glu-6-phosphatase, hence no gluconeogenesis
from G6P.
·
In
Liver:
o
Lactate
is re-converted to pyruvate
for gluconeogenesis.
o
Gluconeogenesis requires
ATP and GTP.
o
The consumed ATP is regenerated through Oxi Pho.
·
Separation
of the ATP-generation glycolysis and the
ATP-consuming gluconeogenesis processes in two
separate organs.
·
The
combination of these two processes in a single cell would be a futile cycle.
Glucose-Alanine Cycle (Fig. 21-7)
·
In
Muscle:
o
Glucose and glycogen (via G6P) enter glycolysis to generate pyruvate
and ATP.
o
Transaminase
allows (AA + Pyruvate ⇌ α–Keto acid + Alanine)
·
Alanine
is transported from muscle to liver via bloodstream
·
In
Liver:
o
Alanine
is re-converted to pyruvate
for gluconeogenesis.
o
Gluconeogenesis requires
ATP and GTP.
o
The consumed ATP is regenerated through Oxi Pho.
o
The alanine amino group is recovered in NH4+
or aspartate à urea
synthesis.
·
Separation
of the ATP-generation glycolysis and the
ATP-consuming gluconeogenesis processes in two
separate organs.
·
This
cycle also allows the transfer of nitrogen from muscle to liver.
HORMONE ACTION:
SIGNAL TRANSDUCTION
In
Animals:
·
Endocrine
glands synthesize and release hormones.
·
Hormones
are carried by bloodstream to target cells.
·
Binding
of hormone to specific receptor on the surface of target cells.
·
The
binding of an extracellular ligand elicits, via the receptor, a discrete
biochemical effect inside of cell. This
is signal transduction.
·
Signal
transduction pathways are very complex, allowing cells to react to discrete
signals (individually or in combination) with variations in the magnitude and
duration of their responses.
Hormones
Control Fuel Metabolism by:
·
Maintaining
homeostasis (e.g. constant blood glucose)
·
Responding
to a wide variety of stimuli (e.g. via epinephrine and norepinephrine)
·
Follow
cyclic and developmental programs (e.g. maturation, sexual differentiation,
etc.)
The Adenylate Cyclase Signaling System
·
Adrenal
gland cortex (outer layer) synthesizes and secretes steroid hormones.
·
Adrenal
gland medulla synthesizes and releases epinephrine and norepinephrine.
·
Epinephrine
and norepinephrine can bind to a class of integral
membrane glycoprotein receptors.
·
These
receptors are structurally typified by (a) an extracellular glycosylated
N-terminal domain, (b) 7 tranmembrane hydrophobic
helices, and (c) an intracellular C-terminal domain.
Mechanism of Adenylate Cyclase System (See Fig. 21-15)
·
Binding
of hormone to extracellular glycosylated receptor
site.
·
Conformational
change of the receptor protein intracellular C-terminal domain
·
The
receptor intracellular domain, in its new conformation, interacts with an abg heterotrimeric G-protein (Gs, or G, or s in Fig.
21-15) for its activation.
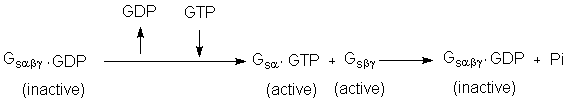
·
Gsα•GTP activates plasma membrane-bound adenylate cyclase.
·
Formation
of intracellular cAMP – a “second messenger”
·
Binding
of cAMP to inactive cAMP-dependet
protein kinase R2C2 induces the dissociation of 2 active
C from R2:cAMP.
·
Phosporylation of Ser or Thr residues of
cellular proteins for specific responses.
·
Gsα
is an GTPase, allowing
turnover of active Gsα•GTP
to Gsα•GDP
and subsequent formation of inactive Gsαbg•GDP.
Regulation via
Inhibitory G-Protein
·
An
inhibitory G-protein (Gi) offers regulation following
a similar set of events except that Giα•GTP inactivates adenylate cyclase.
Receptor Tyrosine Kinases
·
Many
protein hormones known
as growth factors stimulate the proliferation and differentiation of target
cells by binding to Receptor Tyrosine Kinases (RTKs).
·
Structurally
they are typified by (a) an extracellular hormone binding domain, (b) a single
trans-membrane domain, and (c) an intracellular tyrosine kinase domain.
Mechanism of RTKs
·
Usually
RTKs exist as monomers. Example: human growth hormone receptor.
·
A
single hormone molecule binds to two monomeric RTKs to form a complex.
·
The
complex formation of the two intracellular domains actives the tyrosine kinase
activity.
·
Results
in mutual cross-phosphorylation of Tyr residues of the kinase domains.
·
This
autophosphorylation activates Tyrosine Kinase activity, enabling subsequent phosphorylation of
tyrosine residues of intracellular proteins.
·
The
activated RTK can also bind certain cytoplasmic
protein tightly, via interactions between a SH2 domain
of the latter with the phosphorylated tyrosine
residues of RTK.
·
This
binding leads to phosphorylation of the cytoplasmic protein or a change of its conformation to
trigger a subsequent sequence of cellular events (involving a monomeric G-protein named Ras).
·
Protein
tyrosine phosphatases serve as an “off switch” for RTKs.
The Dimeric Insulin
Receptor
·
A
transmembrane glycoprotein with tyrosine kinase
activity.
·
In
the unliganded state, it exists as a dimer of two (αβ) monomers. (Fig. 21-16)
·
The
α is entirely
in the extracellular space and the β spans through the membrane.
·
When
insulin binds to two α subunits, a conformational change occurs in the
intracellular domain of β, enabling autophosphorylations
and activations of tyrosine kinase activity.
·
The
activated kinase domain does not interact directly with any SH2-containing
proteins.
·
However,
the activated kinase domain can bind and phosphorylate
a number of proteins, mainly the insulin receptor substrate-1 and 2 (IRS-1 and
IRS-2).
·
The
phosphorylated IRS-1 and IRS-2 can then bind certain
SH2-containing proteins.
The Phosphoinositide
Pathway (Fig. 21-22)
·
Phosphatidylinositol-4,5-bisphosphate (PIP2), a minor structural component of
plasma membranes, is a part of a second messenger system.
·
Binding
of exacellular ligand to a
receptor (also containing a 7 transmembrane
segments) enables the inateraction with an αβg hetertrimeric
G-protein (Gq or q in diagram).
·
Formation
of active qα•GTP, which activates a
membrane-bound phospholipase C (PLC).
·
Enabling
hydrolysis of PIP2 to form inositol-1,4,5-triphosphate (IP3) and
1,2-diacylglycerol (DAG)
·
IP3
is a water-soluble second messenger:
o Binds to and induces the opening of a
Ca2+ transport channel in endoplasmic reticulum, allowing efflux of
Ca2+ to significantly increase cytosolic
[Ca2+].
o Triggers diverse cellular responses.
·
DAG
is a lipid-soluble second messenger:
o Remains in plasma membranes
o Activates several cellular proteins.