|
FATE
OF C SKELETON |
·
Standard
AAs are degraded to one of 7 metabolic
intermediates: pyruvate;
a-ketoglutarate; succinyl-CoA; fumarate; oxaloacetate; acetyl-CoA; or acetoacetate.
·
Fig.
20-13
·
In
animals, Leucine and Lysine are the only two purely ketogenic
AAs (i.e. they can be converted to acetoacetate or acetyl-CoA; no
net synthesis of pyruvate or any of the TCA intermediates).
·
Five
AAs (Isoleucine; Threonine; Phenylalanine;
Tyrosine; Tryptophan) are both glucogenic (i.e. they are first converted to pyruvate or any of the TCA
intermediates) and ketogenic.
·
13 other AAs are purely glucogenic.
|
KETOGENESIS |
(Chapter 19, section
3, pp. 649-650. Fig. 19-21)
·
A
mitochondrial process by which acetyl-CoA is
converted to acetoacetate and D-β-hydroxybutyrate.
·
Ketone Bodies: acetoacetate, D-β-hydroxybutyrate,
and acetone.
·
Ketone bodies are water-soluble equivalents of fatty acids.
·
Important
metabolic fuels for peripheral tissues, especially heart and skeletal muscle.
·
The
brain utilizes glucose for energy under normal circumstances. However, ketone
bodies become brain’s major fuel source during starvation.
A. Formation of Acetoacetate in Mitochondria
·
Thiolase
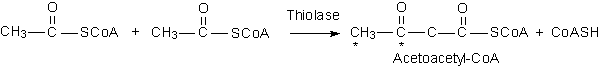
·
Hydroxymethylglutaryl-CoA synthase b-Hydroxymethylglutaryl-CoA
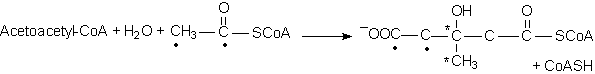
(Corresponding
enzymes also exist in cytosol which, together with HMG-CoA reductase,
function in mevalonate synthesis.)
·
HMG-CoA Lyase
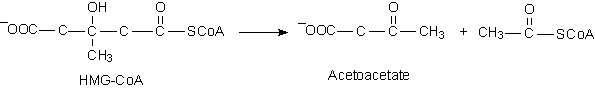
B.
Formation of D-β-Hydroxybutyrate
·
β-Hydroxybutyrate
Dehydrogenase
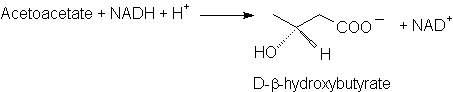
Note:
·
FAs
β-Oxidation:
enoyl-CoA hydratase 3-L-hydroxyacyl-CoA
DH
trans-∆2-enoyl-CoA → L-β-hydroxyacyl-CoA → β-ketoacyl-CoA
·
FAs
synthesis:
β-ketoacyl-ACP reductase β-hydroxyacyl-ACP dehydrase
β-ketoacyl-ACP
→
D- β-hydroxyacyl-ACP →
trans-∆2-enoyl-ACP
C. Formation of
Acetone (by
a facile nonenzymatic reaction)
![]()
=======================================================
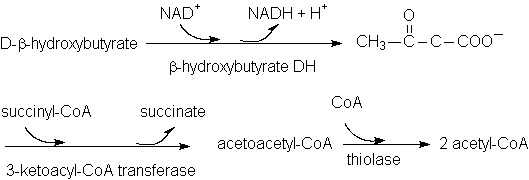
Utilization of Ketone Bodies (Fig. 19-22)
Liver releases acetoacetate and β-hydroxybutyrate
→ carried by blood stream
→ peripheral tissues → converted to acetyl-CoA and used as fuels.
|
TETRAHYDROFOLATE AND AA
METABOLISM |
·
Structure of Tetrahydrofolate (p.
704)
· Conversion of Folate D Dihydrofolate D Tetrahydrofolate (Fig. 20-19)
·
Oxidation
Levels of C1 Groups Carried by THF (Table 20-2)
·
Consider “N”
the equivalent of “O”
Methanol HO–CH3 =N–CH3 N5-Methyl-THF
Formaldehyde H2C=O =N–CH2–N= N5,N10-Methylene-THF
Formate HC(OH)=O =N–CH=O N5-Formyl-THF; N10-Formyl-THF
=N–CH=NH N5-Formiminol-THF
=N+=CH–N= N5,N10-Methenyl-THF
Interconversion of
the C1 Units Carried by THF (Fig. 20-20)